




In conjunction with the development of Medical Technology, Medical Devices are increasingly diverse. They range in scope from a Q-tip to an X-ray machine and the manufacturing process involves uncountable applications. PROSTECH is confident to keep pace with medical technology development and provide customers with the utmost medical adhesive solutions.
Firstly, our range of adhesives for medical devices is equally as diverse and has been carefully selected to demonstrate our capabilities. There are a few key features of the popular products. However, the effectiveness we mentioned will be reflected in our customizing product line which can be formulated to best suit your requirements.
Furthermore, we do not promise to provide the best adhesive Adequate Adhesive Solution. Some medical device adhesive applications require adhesives formulated to pass biocompatibility testing (not all medical devices), others require structural bonding of equipment. It is more important to select the right adhesive rather than the best one.
In Medical Devices Manufacturing Industry, whether you are seeking a unique UV curable adhesive to bond a state-of-the-art oxygenator or a structural bond of an MRI housing we are here to help.
Some main medical devices requirement and standards for adhesive

ISO 10993 biocompatibility standards (ISO 10993-5,-10,-11,-4,-6)
Depending on the application requiring an adhesive solution, many of our products also have passed ISO 10993 testing for skin sensitization and irritation as well.

ISO 13485 Medical Devices Quality Management
Ensuring that our products are made consistently and that they will conform to Medical Device Quality Management Standards.

USP Class VI Bio-compatibility of Materials
set by the US Pharma Convention (USP) with a focus on medications, healthcare technologies, food ingredients, and materials used in medical devices.

FDA Approval for Medical Devices
Since the user must submit his part for final testing and FDA certification, an adhesive’s qualification to ISO 10993 or USP Class VI is viewed as sufficient to allow a user to determine “probable” device acceptance.
Detailed Application
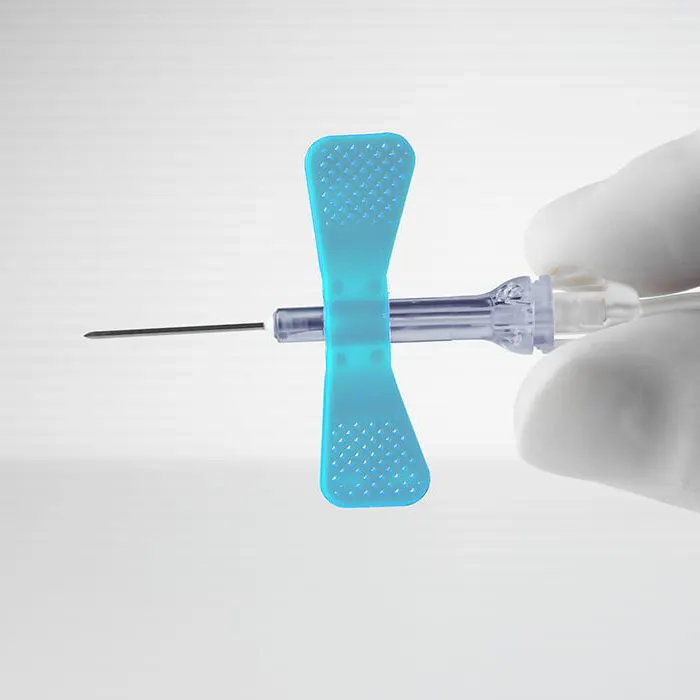

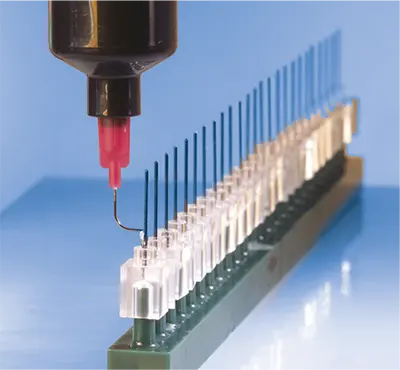
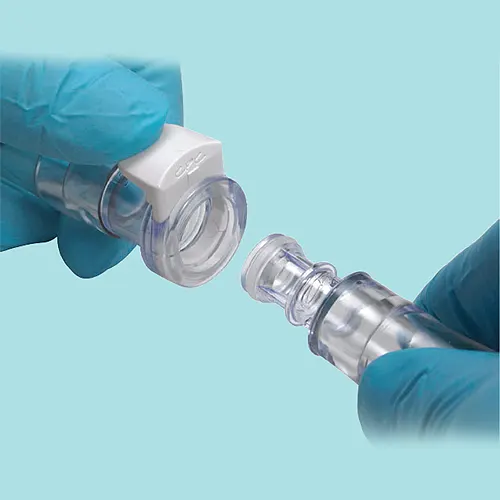
Useful Information

