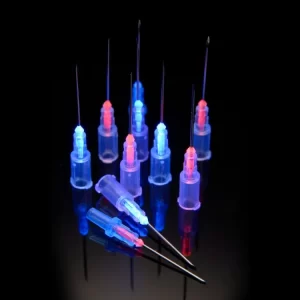
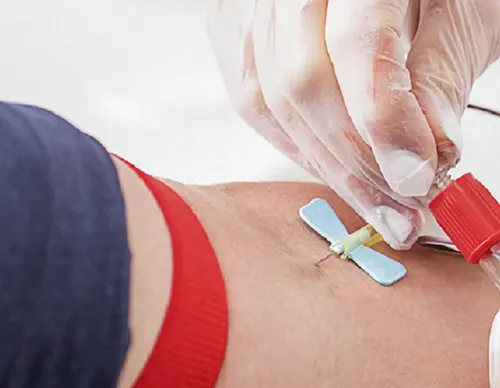
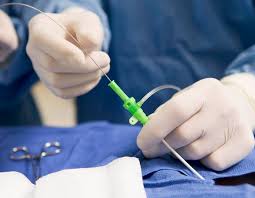
Adhesives are universally used for bonding cannulae to hubs in needle assemblies. It is critical that this joint is well sealed to prevent fluids, such as blood or medicine, from leaking. It is also essential that the position of the cannula remains fixed. Since the adhesive joint has a profound and visible impact on the fitness-of-use of the needle assembly, needle designers often specify large safety factors on the adhesive joint, while their manufacturers operate with Six Sigma control limits. These stringent requirements cause the adhesive selection and qualification process to be lengthy and expensive.
To solve this problem, we are confident to introduce our assembly solution and the one-stop guideline as follows:
Adhesive
The three most commonly used adhesive chemistries for needle manufacturing are:
- Light Cure Acrylics (both UV cure and LED cure)
- Light Cure Cyanoacrylates
- One-Part Heat Cure Epoxies
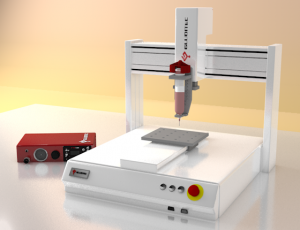
Automated Dispensing System for Single Part Adhesive
Though the benefits of fast-setting adhesives, these moisture-cure adhesives can be a challenge, especially when your assembly process requires precise, repeatable dispensing. See details of the Different Dispensing Systems
UV or Heat Curing System
Because UV light of varied spectral output penetrates glass, polypropylene, and many other plastics, both flat and complex 3D-shaped parts are easily bonded instantaneously. By ensuring superior bonding at higher speeds, UV curing produces quality assemblies faster and less expensively. UV Curing System Details here
Useful Information
Is Your Adhesive Biocompatible?
Biocompatibility is a general term used to describe the property of material being compatible with living tissue or living system.
Medical Adhesive and Typical Application in Medical Industry
Our Medical Device Adhesives are relied upon to increase output and improve efficiency when producing medical and assistive devices. A